Catalysts Are Effective Because They . explain how enzymes function as molecular catalysts. a catalyst has no effect on the solution equilibrium of a reaction, it increases the rate of approach to equilibrium. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Adding a drop of blood. A substance that helps a chemical reaction. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; a fundamental task of proteins is to act as enzymes —catalysts that increase the rate of virtually all the chemical reactions within cells. Discuss enzyme regulation by various factors. catalysts are compounds that speed up chemical reactions by lowering the energy barrier between reactants and products. catalysts can speed up chemical reactions because they have the ability to change how reactants turn into products. an important principle is that since they only reduce energy barriers between products and reactants, enzymes always catalyze.
from www.mdpi.com
a fundamental task of proteins is to act as enzymes —catalysts that increase the rate of virtually all the chemical reactions within cells. explain how enzymes function as molecular catalysts. catalysts are compounds that speed up chemical reactions by lowering the energy barrier between reactants and products. Adding a drop of blood. catalysts can speed up chemical reactions because they have the ability to change how reactants turn into products. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. a catalyst has no effect on the solution equilibrium of a reaction, it increases the rate of approach to equilibrium. A substance that helps a chemical reaction. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; Discuss enzyme regulation by various factors.
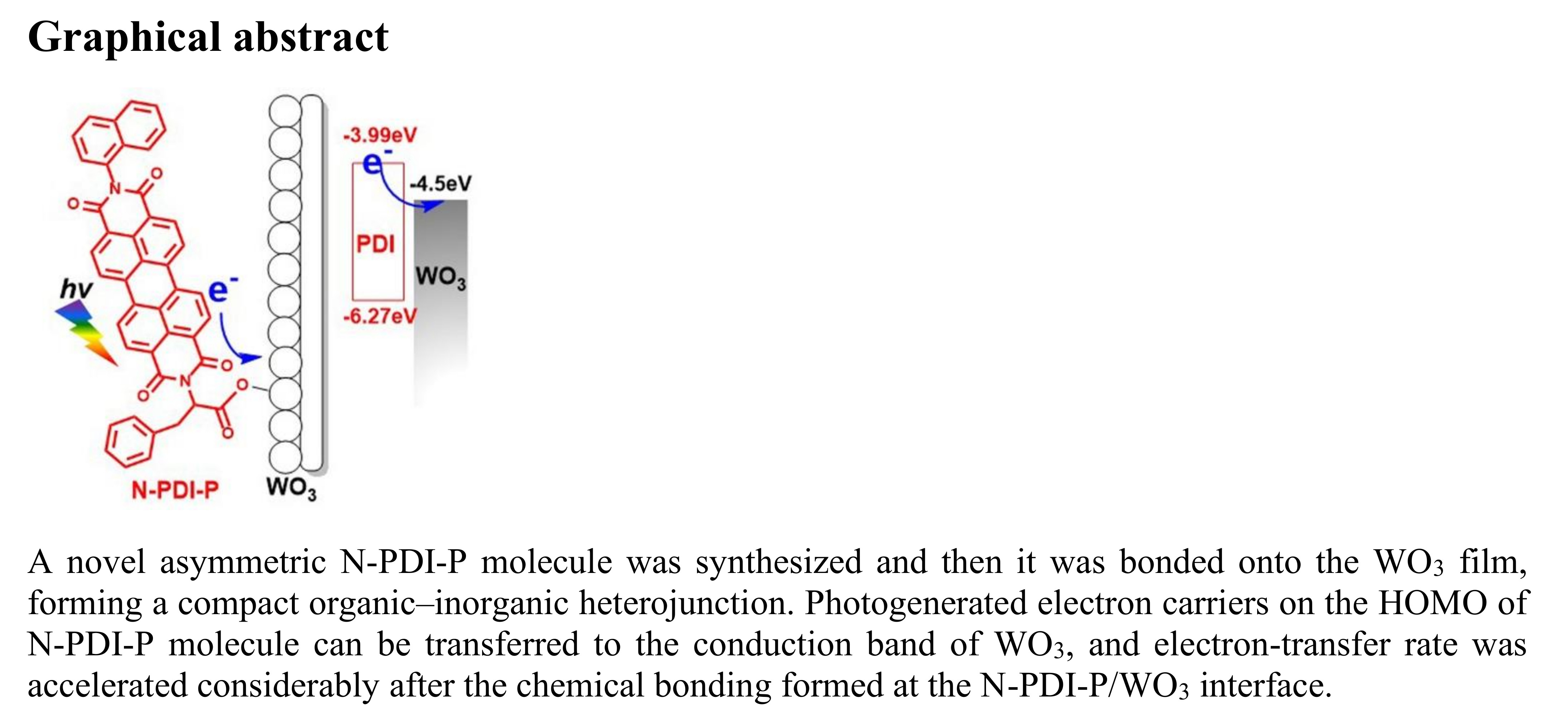
Catalysts Free FullText Chemically Bonded NPDIP/WO3 Organic
Catalysts Are Effective Because They explain how enzymes function as molecular catalysts. an important principle is that since they only reduce energy barriers between products and reactants, enzymes always catalyze. Adding a drop of blood. A substance that helps a chemical reaction. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; Discuss enzyme regulation by various factors. catalysts are compounds that speed up chemical reactions by lowering the energy barrier between reactants and products. catalysts can speed up chemical reactions because they have the ability to change how reactants turn into products. a catalyst has no effect on the solution equilibrium of a reaction, it increases the rate of approach to equilibrium. a fundamental task of proteins is to act as enzymes —catalysts that increase the rate of virtually all the chemical reactions within cells. explain how enzymes function as molecular catalysts.
From www.mdpi.com
Catalysts Free FullText Recent Advances in gC3N4Based Catalysts Are Effective Because They catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. A substance that helps a chemical reaction. a catalyst has no effect on the solution equilibrium of a reaction, it increases the rate of approach to equilibrium. an important principle is that since they only reduce energy barriers. Catalysts Are Effective Because They.
From www.chegg.com
Solved Part 1. Enzymes are potent catalysts because they a) Catalysts Are Effective Because They catalysts are compounds that speed up chemical reactions by lowering the energy barrier between reactants and products. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; explain how enzymes function as molecular catalysts. catalysts can speed up chemical reactions because they have the ability to change how reactants turn. Catalysts Are Effective Because They.
From www.researchgate.net
(a) Comparison of the catalytic activities of different supported Pd Catalysts Are Effective Because They the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; a fundamental task of proteins is to act as enzymes —catalysts that increase the rate of virtually all the chemical reactions within cells. Adding a drop of blood. explain how enzymes function as molecular catalysts. catalysts are compounds that speed. Catalysts Are Effective Because They.
From www.mdpi.com
Catalysts Special Issue Metal Catalysts Recycling and Heterogeneous Catalysts Are Effective Because They catalysts are compounds that speed up chemical reactions by lowering the energy barrier between reactants and products. Adding a drop of blood. catalysts can speed up chemical reactions because they have the ability to change how reactants turn into products. explain how enzymes function as molecular catalysts. the most effective catalyst of all is the enzyme. Catalysts Are Effective Because They.
From www.mdpi.com
Catalysts Free FullText Bulky NHCCobalt ComplexCatalyzed Highly Catalysts Are Effective Because They Discuss enzyme regulation by various factors. catalysts can speed up chemical reactions because they have the ability to change how reactants turn into products. a fundamental task of proteins is to act as enzymes —catalysts that increase the rate of virtually all the chemical reactions within cells. Adding a drop of blood. catalysts are compounds that speed. Catalysts Are Effective Because They.
From www.mdpi.com
Catalysts Free FullText Oxidative Coupling of Methane over Mn2O3 Catalysts Are Effective Because They a catalyst has no effect on the solution equilibrium of a reaction, it increases the rate of approach to equilibrium. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. an important principle is that since they only reduce energy barriers between products and reactants, enzymes always catalyze.. Catalysts Are Effective Because They.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalysts Are Effective Because They catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. catalysts are compounds that speed up chemical reactions by lowering the energy barrier between reactants and products. Adding a drop of blood. catalysts can speed up chemical reactions because they have the ability to change how reactants turn. Catalysts Are Effective Because They.
From www.mdpi.com
Catalysts Free FullText Review of Heterogeneous Catalysts for Catalysts Are Effective Because They catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. catalysts can speed up chemical reactions because they have the ability to change how reactants turn into products. Adding a drop of blood. a fundamental task of proteins is to act as enzymes —catalysts that increase the rate. Catalysts Are Effective Because They.
From www.mdpi.com
Catalysts Free FullText Highly Efficient Catalytic Reduction of Catalysts Are Effective Because They a catalyst has no effect on the solution equilibrium of a reaction, it increases the rate of approach to equilibrium. an important principle is that since they only reduce energy barriers between products and reactants, enzymes always catalyze. Adding a drop of blood. a fundamental task of proteins is to act as enzymes —catalysts that increase the. Catalysts Are Effective Because They.
From www.mdpi.com
Catalysts Free FullText Switchable StimuliResponsive Catalysts Are Effective Because They Adding a drop of blood. a fundamental task of proteins is to act as enzymes —catalysts that increase the rate of virtually all the chemical reactions within cells. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. catalysts can speed up chemical reactions because they have the. Catalysts Are Effective Because They.
From slideplayer.com
Transition metals catalysts Catalysts lower activation energies Catalysts Are Effective Because They a fundamental task of proteins is to act as enzymes —catalysts that increase the rate of virtually all the chemical reactions within cells. an important principle is that since they only reduce energy barriers between products and reactants, enzymes always catalyze. catalysts are compounds that speed up chemical reactions by lowering the energy barrier between reactants and. Catalysts Are Effective Because They.
From www.mdpi.com
Catalysts Free FullText Chemically Bonded NPDIP/WO3 Organic Catalysts Are Effective Because They explain how enzymes function as molecular catalysts. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Discuss enzyme regulation by various factors. a fundamental task of proteins is to act as enzymes —catalysts that increase the rate of virtually all the chemical reactions within cells. a. Catalysts Are Effective Because They.
From www.slideserve.com
PPT Enzymes as Biological Catalysts PowerPoint Presentation ID591293 Catalysts Are Effective Because They A substance that helps a chemical reaction. catalysts are compounds that speed up chemical reactions by lowering the energy barrier between reactants and products. catalysts can speed up chemical reactions because they have the ability to change how reactants turn into products. Adding a drop of blood. a catalyst has no effect on the solution equilibrium of. Catalysts Are Effective Because They.
From www.mdpi.com
Catalysts Free FullText Classification, Synthetic, and Catalysts Are Effective Because They explain how enzymes function as molecular catalysts. Discuss enzyme regulation by various factors. Adding a drop of blood. a catalyst has no effect on the solution equilibrium of a reaction, it increases the rate of approach to equilibrium. catalysts are compounds that speed up chemical reactions by lowering the energy barrier between reactants and products. a. Catalysts Are Effective Because They.
From www.mdpi.com
Catalysts Free FullText Advances in Catalytic of N2O Catalysts Are Effective Because They catalysts can speed up chemical reactions because they have the ability to change how reactants turn into products. an important principle is that since they only reduce energy barriers between products and reactants, enzymes always catalyze. a fundamental task of proteins is to act as enzymes —catalysts that increase the rate of virtually all the chemical reactions. Catalysts Are Effective Because They.
From www.mdpi.com
Catalysts Free FullText General and Prospective Views on Oxidation Catalysts Are Effective Because They Adding a drop of blood. a fundamental task of proteins is to act as enzymes —catalysts that increase the rate of virtually all the chemical reactions within cells. Discuss enzyme regulation by various factors. a catalyst has no effect on the solution equilibrium of a reaction, it increases the rate of approach to equilibrium. catalysts are compounds. Catalysts Are Effective Because They.
From www.mdpi.com
Catalysts Free FullText Engineering Surface Ligands of Noble Metal Catalysts Are Effective Because They a catalyst has no effect on the solution equilibrium of a reaction, it increases the rate of approach to equilibrium. Adding a drop of blood. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. catalysts are compounds that speed up chemical reactions by lowering the energy barrier. Catalysts Are Effective Because They.
From 2012books.lardbucket.org
Catalysis Catalysts Are Effective Because They a fundamental task of proteins is to act as enzymes —catalysts that increase the rate of virtually all the chemical reactions within cells. explain how enzymes function as molecular catalysts. Discuss enzyme regulation by various factors. A substance that helps a chemical reaction. the most effective catalyst of all is the enzyme catalase, present in blood and. Catalysts Are Effective Because They.